Description
Objectives and expected results
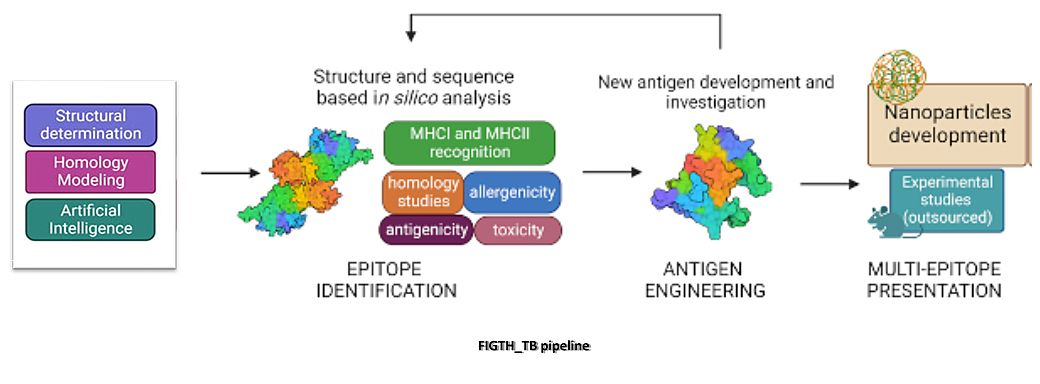
The scientific mission of PRIN 2022 PNRR-FIGHT_TB is to identify, structurally characterize to engineer new antigens for the development a multi-antigenic molecules against TB. To achieve this mission, IBB will make massive use of molecular and structural biology (structural vaccinology approach), albeit in a multidisciplinary network including computational and organic chemistry, to characterize Mtb antigens altready studied at IBB for the definition of a new multi-antigenic vaccine. Indeed, functional and structural information will help to understand the key role of identified antigens and allow a rational design of these molecules antigens with the aim to improve their antigenic features. Also, computational approaches and well-defined bioinformatics tools help the design of improved antigens. These analyses will provide good starting points for the conjugation of different antigens in order to elicit effective and simultaneous immune responses at different levels.
To this aim, the most immunodominant peptides or protein fragments will be linked together to develop a multi-antigenic chimera, using both synthetic and recombinant technologies. The best hits will be then used to develop ad hoc nanoparticles as platform to exploit the principle of antigen presentation.
Project proponents
- Istituto di Biostrutture e Bioimmagini-CNR
Involved entities
- Dipartimento di Farmacia/DIFARMA della Università degli Studi di Salerno (UNISA)

